News attention
05
2020
-
02
Scientific Research News | Research Progress of novel coronavirus Pneumonia (COVID-19) (V)
1. 2019-nCoV Biology Research Progress
2020 Feb 2
Shi Zhengli's team from the Wuhan Institute of Virology, Chinese Academy of Sciences, published a research paper entitled "A pneumonia outbreak associated with a new coronavirus of probable bat originn" online in Nature. The paper notes that early in the outbreak, the research team obtained full-length genome sequences from 5 patients, which were almost identical to each other, with a total of 79.5% of the sequences recognizing SARS-CoV. Two-pair sequence analysis of 7 conserved non-structural proteins revealed that the virus belonged to the bat SARSr-CoV species. The 2019-nCoV virus was isolated from the bronchoalveolar lavage fluid of a critically ill patient and was neutralized by the serum of several patients. Finally, the research team found that the sequence of the new coronavirus nCoV-2019 ravaging Wuhan was up to 96% consistent with the sequence of a bat coronavirus at the genome-wide level! Importantly, the team has confirmed that this new CoV uses the same cells as SARS-CoV to enter the receptor ACE2. The results of this study provide an important basis for subsequent research on the pathogenic mechanism of virus and virus traceability.
Zhang Yongzhen's team from Fudan University published a research paper titled "A new coronavirus associated with human respiratory disease in China" in Nature Online, which reported a patient working in a seafood market who was admitted to Wuhan Central Hospital on December 2019, 12, with severe respiratory syndrome, including fever, dizziness and cough. Genomic RNA sequencing of bronchoalveolar lavage fluid samples identified a novel RNA coronavirus, referred to here as WH-Human-26 coronavirus (also known as 1-nCoV). Phylogenetic analysis of the complete viral genome (2019,29 nucleotides) revealed that the virus was most similar to a group of SARS-like coronavirus samples from bats in China (903.89% nucleotide similarity). The outbreak highlights the virus's ability to spread from animals to humans and cause severe disease.
The research paper published online by Yi Li's team from the Wuhan Institute of Bioengineering estimates that the 2019-nCoV outbreak may have originated in Wuhan on November 2019, 11, and Wuhan is the main hub for the spread of the 9-nCoV outbreak in China and beyond. The findings may be useful for effective prevention strategies involving 2019-nCoV in China and beyond. However, due to the limited number of 2019-nCoV genome sequences, this result is estimated to be somewhat uncertain. Therefore, the conclusions should be considered preliminary and should be interpreted with caution. As the pandemic continues, adding more new sequences to the analysis could significantly alter these results. The findings suggest that the 2019-nCoV outbreak was caused by migration and travel, so local, national, and international strategies must be considered when designing interventions to end the spread of 2019-nCoV in China and beyond.
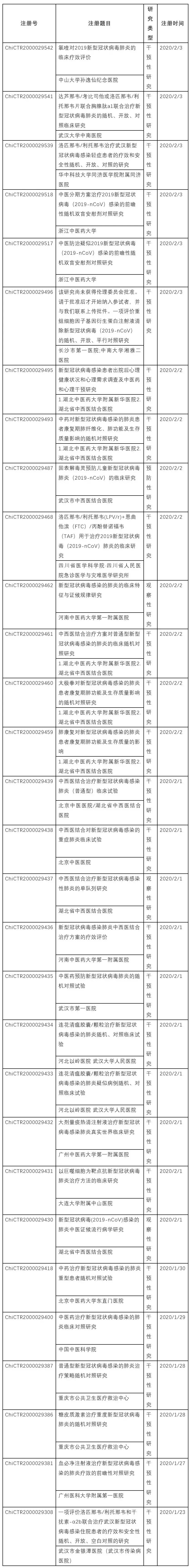
According to Thailand's Ministry of Health, a Chinese woman infected with the novel coronavirus experienced significant improvement in her condition after being treated with an antiviral cocktail therapy used to treat influenza and HIV. Doctor Kriengsak Attipornwanich said the 71-year-old patient tested negative for the novel coronavirus 48 hours after receiving this combination treatment. Doctors combine the anti-flu drug oseltamivir with the antiviral drugs lopinavir and ritonavir used to treat HIV. It should be emphasized that doctors say that whether this treatment can be rolled out to more patients will need to wait for further research. In addition, according to Chinese scientists reported in The Lancet, Wuhan Jinyintan Hospital has initiated a randomized controlled trial (ChiCTR2019) of the combination of lopinavir and ritonavir for the treatment of pneumonia infected with 2000029308-nCoV. The trial aimed to evaluate the efficacy and safety of the lopinavir/ritonavir and interferon-alpha2b combination in the treatment of hospitalized patients with novel coronavirus infection in Wuhan. A search in the China Clinical Trials Registry (www.chictr.org.cn) found that on February 2, the Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital also launched a related clinical study (ChiCTR2) to evaluate the efficacy of lopinavir/ritonavir (LPV/r) + emtricitabine (FTC)/tenofovir propofovir (TAF) for the treatment of 2000029468 novel coronavirus (2019-nCoV) pneumonia. Including the above two domestic studies, the China Clinical Trials Registry has registered and launched 2019 novel coronavirus studies by February 2, involving a number of traditional Chinese and western medicines. The drugs involved in the trial include traditional Chinese medicine, Xuebijing injection, glucocorticoids, Lianhua Qingwen capsules/granules, ribavirin + interferon α-3b, lopinavir/ritonavir + interferon-α30b, etc., see Appendix 1: Novel coronavirus research projects registered and launched by the China Clinical Trials Registry.
At the press conference in Hubei Province, Wang Wei, director of the Department of Science and Technology of Hubei Province, revealed that in terms of animal traceability research, studies confirmed that the new coronavirus uses the same cells as SARS coronavirus to enter the receptor, and the study found that the coronavirus sequence consistency of the new coronavirus and a bat is as high as 96%.
A news report published in the top international journal Nature said virologists around the world are eager to obtain live samples of 2019-nCoV and are developing plans to test vaccines and drugs, build animal models and explore the biology associated with the virus.
2. 2019-nCoV therapeutic drug research progress
On January 2020, 1, the team of Yu Wenying, an associate researcher at the Institute of Pharmaceutical Sciences, China Pharmaceutical University, changed the traditional research idea of "targeting drug finding" and innovated the screening method, that is, taking the existing active SARS inhibitor proven by X-RAY single crystal diffraction as a template, using a ligand-based drug design method, drug screening among all listed drugs, and quickly discovered a variety of drugs that may be effective against the new coronavirus, which have potential clinical application value. Through specific analysis, Yu Wenying's team found 31 broad-spectrum antiviral drug and 1 anti-HIV protease inhibitors, 3 ACE inhibitor antihypertensive drug, 1 antibacterial drug for the treatment of tuberculosis and 1 broad-spectrum antibacterial drugs. These drugs, alone or in combination, can provide a reference for the clinical treatment of patients with novel coronavirus pneumonia. They will continue to carry out targeted anti-novel coronavirus drug activity tests in depth, in order to provide more powerful guidance for clinical research and treatment.
(Please refer to: http://news.cpu.edu.cn/eb/72/c243a125810/page.htm)
On February 2020, 2, after U.S. health officials confirmed the possibility of a global pandemic of the novel coronavirus, GlaxoSmithKline (GSK), the world's vaccine giant, and the Coalition for Epidemic Preparedness Innovations (CEPI) jointly announced that it would provide its AS2 adjuvant technology to enhance the 03-nCoV vaccine research and development program funded by CEPI. GSK's adjuvants are already used in vaccines around the world, including the H2019N1 and H1N5 pandemic influenza vaccines. It helps enhance the efficacy of vaccines, thereby helping researchers make vaccines more quickly to feed the global pandemic trend.
(Please refer to: http://www.xinhuanet.com//health/2020-02/04/c_1125528746.htm)
3. 2019-nCoV latest epidemiological statistics
Theoretical evidence for virus evolution (published January 2020, 1)
Domenico Benvenuto et al. used the genetic information of 2019-nCoV, combined with SARS, MERS and SARS-like coronaviruses of bats, to construct an evolutionary tree, and interpreted the evolutionary relationship between these coronaviruses from the perspective of bioinformatics. They found that the SARS-like coronaviruses of bats isolated from 2019-nCoV in 2015 clustered together, suggesting that the coronavirus epidemic may be derived from the SARS-like coronavirus of bats [1].
Bibliography:
[1] D. Benvenuto, A. Ciccozzi, M. Giovannetti, M. Ciccozzi, S. Spoto, S. Angeletti, The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol 2020.
Appendix 1: Novel coronavirus research projects registered and launched by the China Clinical Trials Registry (February 2).
RELATED NEWS







