News attention
09
2020
-
02
Scientific Research News | Research Progress of novel coronavirus Pneumonia (COVID-19) (VIII)
1.2019-nCoV Epidemic Alert
2020 Feb 2
▄ South China Agricultural University held a press conference on the research of the new coronavirus pneumonia epidemic. According to the press conference, the research team from South China Agricultural University and other units has made a breakthrough in the traceability of potential intermediate hosts of the new coronavirus through joint research. Their latest research suggests that pangolins may be intermediate hosts for the novel coronavirus. It is understood that the research team identified pangolins as potential intermediate hosts of the new coronavirus by analyzing more than 1000,70 metagenomic samples; Then, through molecular biological testing, it was revealed that the positive rate of β coronavirus in pangolins was 99%. The virus was further isolated and identified, and the typical coronavirus particle structure was observed under electron microscopy. Finally, through the genomic analysis of the virus, it was found that the sequence similarity between the isolated virus strain and the current infected human strain was as high as <>%. The above results suggest that pangolins may be intermediate hosts for the novel coronavirus. The research results are of great significance to the source prevention and control of this epidemic, and provide a scientific basis for the adjustment of relevant policies for wildlife control.
2020 Feb 2
▄The "FireEye" laboratory, a new coronavirus emergency testing laboratory that can test 1,30 samples per day, officially started trial operation in Wuhan. The "FireEye" laboratory is jointly built by Wuhan Municipal Government, East Lake High-tech Zone, CCCC-1 Aviation Bureau, Shanghai Nori Laboratory and BGI. On January 31, BGI completed the laboratory design, and CCCC-279 officially started the laboratory construction on January 5, and with the full efforts of 1000 employees of CCCC-2 and Shanghai Norui, the main laboratory construction was completed in only 30 days. The core experimental area covers a total area of 2,12 square meters, and is designed in strict accordance with the P<> (biosafety level <>) laboratory, equipped with BGI's high-throughput sequencing overall solutions and equipment; Equipped with <> B<> level biological safety cabinets; The batch automated nucleic acid extraction platform is equipped with <> automated extraction equipment; At the same time, it is equipped with supporting laboratory compartments, sample rooms, reagent storage rooms, office areas, etc., and the daily flux of new coronavirus nucleic acid testing can reach <>,<> people.
The World Health Organization held a press conference at its headquarters in Geneva to announce the launch of a strategic preparedness and response plan (SPRP) by the international community. The strategic plan, which requires an investment of US$6 million, covering February to April 75, aims to prevent the further spread of the coronavirus in China and around the world, and to protect countries and regions with weak health systems.
▄ Research teams from Wuhan University and other units published in Military Medical Research A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (Standard version) Rapid Recommendations for the Diagnosis and Treatment of Pneumonia Infected with the Novel Coronavirus (2019-nCoV) (Standard Version). The guidelines follow the WHO rapid recommendation guidelines methodology, integrate clinical first-line diagnosis and treatment experience, and focus on the etiology, prevention and control, diagnosis, treatment and care of 2019-nCoV [1].
2. 2019-nCoV Biology Research Progress
2020 Feb 2
▄Lan Fei, Cai Jiabin and others from the Institute of Liver Cancer of Zhongshan Hospital affiliated to Fudan University published research papers online in bioRxiv. The paper pointed out that a considerable proportion of SARS and 2019-nCoV patients will experience varying degrees of liver damage in addition to the respiratory system, and the mechanism and significance of this have not been determined. The study used single-cell RNA-seq data from two separate cohorts to assess cell type-specific expression of ACE2 in healthy liver tissues and identified specific expression of the novel coronavirus receptor ACE2 in cholangiocytes, while hepatocytes expressed very low. The results showed that the liver damage in patients with the new coronavirus may be caused by the virus directly binding to ACE2-positive cholangiocytes to cause bile duct dysfunction, or the toxic side effects caused by therapeutic drugs, rather than the virus directly binding to liver cells. These results suggest that healthcare workers who are treating patients with novel coronavirus infection need to be aware of their hepatic responses, especially those related to cholangiocyte function, and the need for special care for patients with novel coronary pneumonia with abnormal liver function [2].
3. 2019-nCoV therapeutic drug research progress
2020 Feb 2
▄A report by researchers from the University of Mainz in Germany in the famous international medical journal The Lancet said that host-directed therapy should be an option in order to reduce the mortality rate of new coronavirus infection. For 2019-nCoV, there is currently no specific antiviral therapy. At present, the main methods of clinical treatment are symptomatic treatment and organ supportive therapy for intensive care of critically ill patients. The unprecedented activities of WHO and other global public health agencies have focused on preventing the spread of the virus, infection control measures, and screening travellers. Rapid funding has been received for vaccine development, however, just like SARS-CoV and MERS-CoV, funding for therapeutic research and development to reduce 2019-nCoV mortality is currently not available. There is an urgent need to pool funding and scientific investment to advance new therapeutic interventions against coronavirus infection. As 2019-nCoV continues to spread and evolve, the number of deaths is growing exponentially, and advancing the development of new treatments is critical to minimizing the number of deaths from 2019-nCoV infection.
( Please refer to: https://doi.org/10.1016/S0140-6736(20)30305-6)
▄ Justin Stebbing's team at Imperial College London published research papers online in The Lancet, a top international medical journal. The study, based on the knowledge graph of BenevolentAI, a large repository of medical information, used machine learning to search and found that baricitinib may have the ability to reduce the ability to infect lung cells [3]. (Baricitinib, CAS 1187594-09-7, is a JAK1/2 tyrosine kinase inhibitor whose marketing application in China (JXHS1800009) has been approved by the National Medical Products Administration (NMPA) for the treatment of moderate to severe rheumatoid arthritis that does not respond adequately to methotrexate.) )
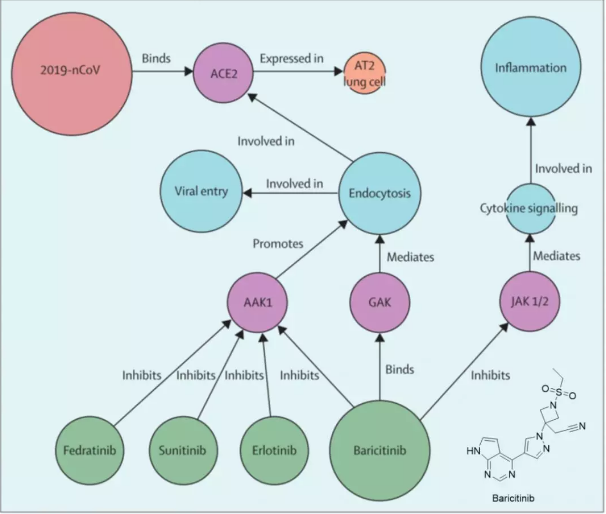
Figure 1. BenevolentAI knowledge graph and Baricitinib's structural formula. The BenevolentAI Knowledge Graph integrates biomedical data from structured and unstructured sources. It uses a series of algorithms to discover new relationships to propose new ways to deal with disease. 2019-nCoV stands for 2019 Novel Coronavirus; AAK1 stands for AP-2-associated kinase 1; GAK stands for cyclin g-associated kinase; JAK1/2 stands for janus kinase 1/2.
▄ Xiao Gengfu, Hu Zhihong, Wuhan Institute of Virology, Chinese Academy of Sciences, and Zhong Wu, Institute of Toxicology and Medicine, Academy of Military Medical Sciences, jointly published research papers online in Cell Research. The study found that chloroquine can effectively inhibit 2019-nCoV infection at the cellular level, but its role in humans has yet to be clinically verified. (Chloroquine, CAS number 54-05-7, blocks viral infection by increasing endosomal pH required for virus/cell fusion; Initially used to treat malaria, its use gradually expanded. In 1951, chloroquine was used to treat rheumatoid arthritis with some effect) Both chloroquine and baricitinib inhibit the entry of the virus into cells, and the two studies are similar [4].
2020 Feb 2
▄The team of Wang Chen and Cao Bin of China-Japan Friendship Hospital announced the launch of remdesivir in the treatment of 2019 new coronavirus infection in Wuhan Jinyintan Hospital, and officially entered clinical trials. A total of 761 patients were enrolled, including 308 patients with mild and moderate disease, and 453 patients with severe disease who were randomly double-blinded for drug testing. Gilead's remdesivir completed a Phase II clinical trial in Australia demonstrating that the drug can inhibit the spread and replication of the Ebola virus, confirming activity against both SARS and Middle East respiratory syndrome (MERS) viral pathogens. These viruses are all coronaviruses and are structurally similar to the 2019-nCoV novel coronavirus. It is worth mentioning that due to the fact that American new crown pneumonia patients took the drug, the effect was significant; Immediately introduced in China, directly skip phase I and phase II clinical trials, and enter phase 3 clinical trials. According to the Remdesivir Phase III clinical trial plan, the trial officially began on February 2, the initial completion will be on April 5, 2020, and the final completion is expected to be on April 4.
4. 2019-nCoV epidemiological statistics
2020 Feb 2
▄ There were 10 new confirmed cases of pneumonia infected by the new coronavirus in Hangzhou, of which 1 couple (husband Xu Moumou and wife Wang Moumou) were diagnosed at the same time and are currently isolated and treated in designated medical institutions. After epidemiological investigation, the confirmed couple in Hangzhou met the confirmed case for 50 seconds in close proximity and were infected without wearing a mask. Another rapid infection in Ningbo was also because the infected person was not wearing a mask. The patient was infected after a brief (about 61 seconds) close proximity to a passerby (confirmed patient in Jiangbei District: female, 15 years old) at the same stall while shopping at Shuangdongfang vegetable market, and neither of them was wearing a mask.
2020 Feb 2
▄ The National Health Commission held a press conference in Beijing. According to reports, the deaths were mainly concentrated in Hubei Province, with a total of 2 people as of 3 o'clock on February 24, accounting for 414% of the country, and the death rate of confirmed cases in Hubei Province was 97.3%, and the national death rate was 1.2%.
▄According to Jiao Yahui, deputy director of the Medical Administration of the National Health Commission, "The number of deaths in Wuhan is 313, accounting for 74% of the national deaths, and the case fatality rate in Wuhan is 4.9%. The case fatality rate in Hubei Province and Wuhan City is higher than the national level, and if the case fatality rate of other provinces except Hubei Province is 0.16%. Therefore, from this set of figures, we can see that the main deaths are still in Hubei, mainly concentrated in Wuhan, for other provinces in the country, although the number of cases is also quite large, but the case fatality rate is actually quite low. From this point of view, we are still confident that the vast majority of our cases are still mild cases, so there is no need to panic.
▄From the analysis of death cases, we have also calculated that the case fatality rate in the country is basically stable, now it is 2.1%, and it was 2.3% at the beginning of the epidemic, which can be said to be a slight decrease. The analysis of death cases, mainly men, accounting for 2/3, women accounted for 1/3, and is mainly of advanced age, more than 80% are over 60 years old, more than 75% are one or more underlying diseases, and these underlying diseases are cardiovascular and cerebrovascular diseases, diabetes, and some patients have basic diseases such as tumors. I want to explain to you that for the elderly who are elderly and have underlying diseases, as long as they are infected with pneumonia, it does not refer to the pneumonia infected by the new coronavirus, these elderly people with underlying diseases, as long as they are infected with pneumonia, it is a high-risk factor clinically speaking, and the case fatality rate itself is also very high, so it is not because of the pneumonia fatality rate infected by the new coronavirus is high."
Bibliography:
[1] Jin Y-H, Cai L, Cheng Z-S, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV)infected pneumonia (standard version). Military Medical Research 2020;7:4.
[2] Chai X, Hu L, Zhang Y, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv 2020:2020.02.03.931766.
[3] Richardson P,GriffinI,TuckerC,etal. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet 2020.
[4] Wang M, Cao R,Zhang L,etal. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020.
Feed | Pingshan Biomedical R&D and Transformation Center, Scientific Research Department
Editor | 鍮 鍮
RELATED NEWS







