News attention
01
2022
-
03
Research Trends | Pingshan Center Achieves High Selective Target Labeling and Drug Release Using Novel Stable Disulfide Bonds
Recently, the research group of Li Zigang/Yin Feng of Pingshan Biomedical Transformation Center of Shenzhen Bay Laboratory published a research paper entitled "Chemo-selective Cys-Pen disulfide for proximity-induced cysteine cross-linking" in the journal ACS Chemical Biology. The paper describes a crosslinking strategy for proximity induced targeting of cysteine using Cys-Pen disulfide bonds, a novel strategy that provides promising chemoselective warheads for the design of covalent inhibitors targeting the side chains of disease-associated protein cysteine.
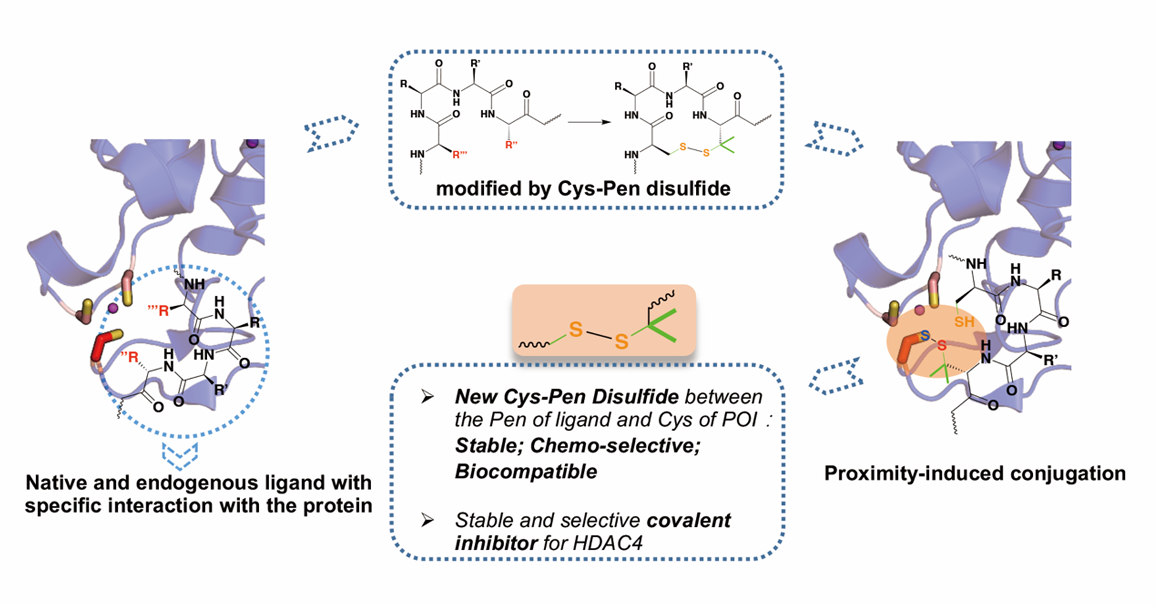
Figure 1 Schematic diagram of Cys-Pen targeting a target in a near-induced covalent manner
Chemicals rich in disulfide bonds are known to be valuable pharmacological tools or therapeutic agents. In addition, ligand-induced coupling strategies offer potential advantages for novel covalent drugs in terms of potency, selectivity, and duration of action. Combining a rich library of disulfide-rich structures and ligand-induced conjugates through thiol-disulfide bond exchange will provide great benefits for the development of site-specific covalent inhibitors. Cys-Cys disulfide bonds are inherently unstable in endogenous reducing environments, while Cys-Pen disulfide bonds exhibit satisfactory stability. The beginning of this paper envisages that the Cys-Pen disulfide bond is a potential ligand-induced covalent bonding warhead that can be reconstituted with protein cysteine near the ligand binding site to form a new disulfide bond. To evaluate the design, PLCγ1-c SH2 and RGS3-PDZ were selected as model proteins for validation. Both proteins are successfully modified by ligands containing Cys-Pen disulfide bonds and new disulfide bonds are formed between the protein and ligand. Subsequent in-depth experiments also proved that the new disulfide bond is a disulfide bond formed between the ligand's Pen and the protein Cys near the ligand binding site. In order to further verify the feasibility of Cys-Pen disulfide bonds as covalent warheads in more complex systems, this paper attempts to design and synthesize a series of peptide inhibitors containing Cys-Pen disulfide bonds targeting the "CCHC" zinc domain near the HDAC4 catalytic pocket, and these inhibitors show satisfactory selectivity and inhibitory effects, further confirming the value of the disulfide bond as a novel cysteine covalent crosslinked warhead.
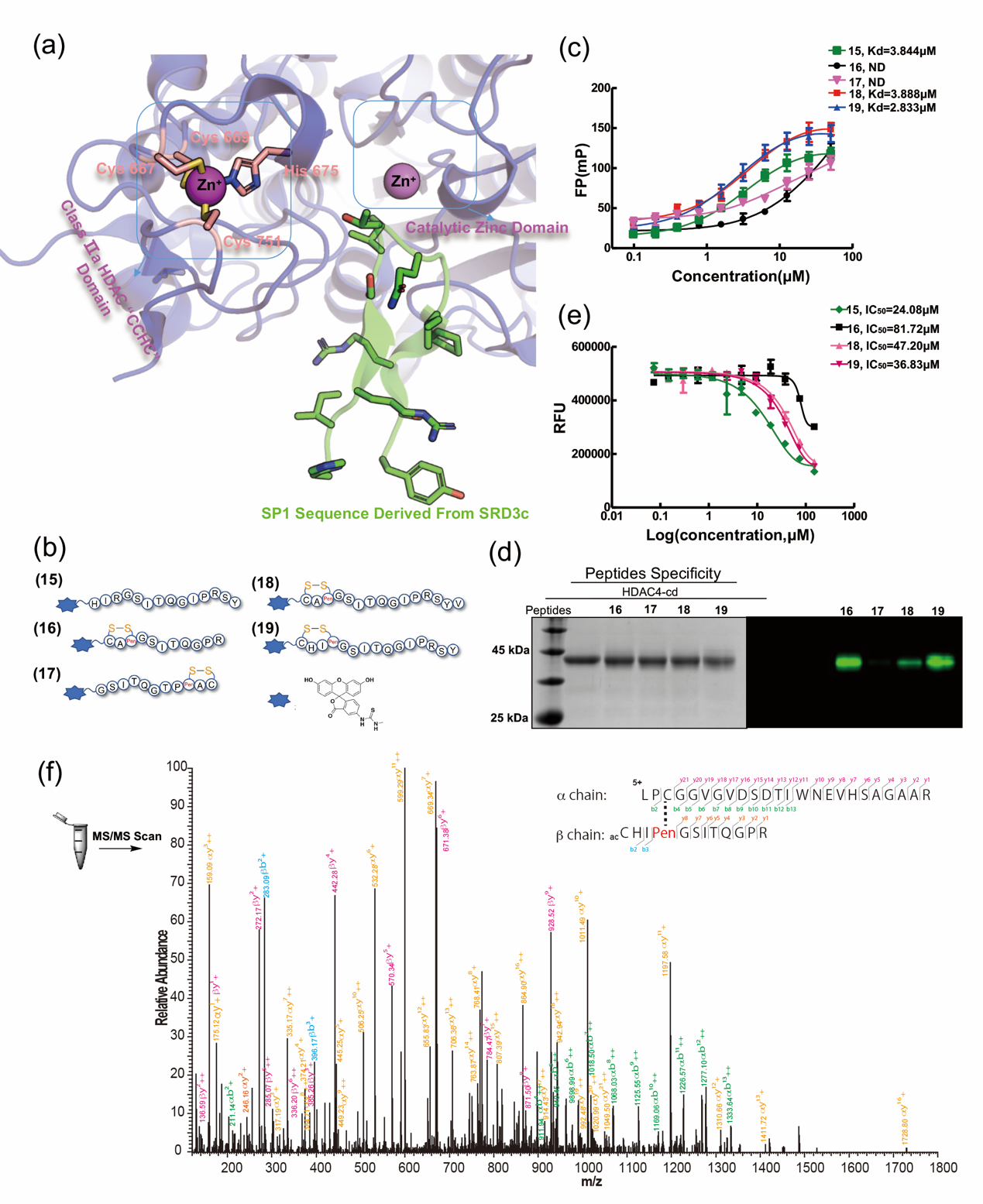
Figure 2 Design strategy and validation experiment of HDAC4-targeting Cys-Pen peptide inhibitor
In addition, it is worth mentioning that the Cys-Pen disulfide bond described here has several important advantages over conventional covalent reactive warheads:
(1) It can tolerate high concentrations of GSH and Trx, and can effectively achieve controllable activation of drugs;
(2) Pen only reacts with Cys near the target surface, and cannot react with amino acids such as Lys, Ser, and Tyr, which can avoid the off-target effect of traditional covalent inhibitors;
(3) Cys-Pen is more stable than Cys-Cys disulfide bonds, which can effectively improve the stability of drugs.
The applicability of this strategy has been illustrated in two important disease-associated proteins.
Due to the relatively high stability of Cys-Pen disulfide bonds, which have not been considered as warheads for the design of covalent inhibitors, this paper uses Cys-Pen disulfide bonds as covalent warheads for the first time, which will provide a promising warhead for the design of less toxic covalent drug molecules, which will also be applicable to many small molecule drugs. In addition, Cys-Pen disulfide bonds may provide new insights into exploring more potential of existing drugs, such as the pan-HDAC inhibitor FK228.
The corresponding authors of this paper are Professor Li Zigang and Professor Yin Feng, Executive Director of Pingshan Biomedical R&D and Transformation Center of Shenzhen Bay Laboratory, Zhan Meimiao, doctoral student of Peking University Advanced Research Institute, Dr. Wang Rui, Dr. Ye Yuxin, Liang Mingchan and Dr. Jiang Chenran of Pingshan Center, Dr. Liu Zhihong of Peking University Advanced Research Institute, doctoral students Liu Na and Zhang Yichi also made important contributions to this paper. The paper has been supported by the National Natural Science Foundation of China, the National Key R&D Program "Synthetic Biology", the Natural Science Foundation of Guangdong Province, the Shenzhen Science and Technology Innovation Commission, the Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Basic Research Institute, the Major Plan of Shenzhen Bay Laboratory and the Shenzhen Bay Laboratory Open Program.
Full text link: https://doi.org/10.1021/acschembio.2c00083
Li Zigang's research group homepage: https://scbb.pkusz.edu.cn/info/1015/1012.htm
Shenzhen Bay Lab: https://www.szbl.ac.cn/scientificresearch/researchteam/68.html
Shenzhen Bay Lab Recruitment https://www.szbl.ac.cn/careers/recruitment/
Shenzhen Bay Laboratory Pingshan Biomedical R&D and Transformation Center Recruitment: http://www.szbl.ac.cn/careers/recruitment/?unit=10
Written by | Peking University Institute of Advanced Research Zhan Meimiao
Editor | Qiu Tian
RELATED NEWS







